Virtual MR-colonography
Jörg F.Debatin, M.D.
Institute of Diagnostic Radiology - University Hospital Essen
Recent improvements in Magnetic Resonance Imaging (MRI) hardware have provided the bases for acquiring 3-dimensional (3D) MRI data sets within a convenient breathhold. In combination with the intravenous application of paramagnetic contrast, breathheld 3D MRI has been shown to be particularly useful in the depiction of arterial morphology (1). The 3D nature of the data acquisitions permits exploitation of a host of image post-processing techniques, permitting analysis from an ‘outer’ as well as an ‘inner’ perspective (2). The latter provides ‘virtual’ endoscopic images. We have adapted this technique for displaying the colon. The technique’s performance was initially assessed in ‘in vitro’ experiments, using human colonic specimens with simulated polypoid lesions. Following an optimisation process, the technique was transposed to patients refered for routine conventional colonoscopy to rule out a colorectal mass lesion. Conventional colonoscopy was performed within 60 minutes of the MRI examination.
Technique
For the MR-exam the patient is placed in a prone position onto the MRI table. A contrast enema is administered per rectum using a disposable enema kit with an inflatable tip (E-Z-EM Inc., Westbury; N.Y.; USA) by hydrostatic pressure (1-1.5 m water column). The enema consists of 2000 ml of water spiked with 20 ml of a 0.5 molar Gd-DTPA solution (Magnevist; Schering AG; Berlin). To reduce bowel motion, scopolamine (20 mg) is administered iv as the patient was placed on the MR table.
Imaging is performed on a 1.5 T MR scanner (Signa EchoSpeed, GE Medical Systems, Milwaukee, Wi), equipped with ultrafast gradients. Filling of the colon is monitored with a 2D acquisition, collecting one image every second. Once contrast has reached the cecum, a 3D Fourier transform gradient echo sequence with spoiling gradients (3) is used to image the colon (TR/TE = 4.7/2 ms, 40° flip angle, 32 cm FOV, 256 x 160 matrix, 0.5 NEX). with 2 mm slice-thickness rendered a voxel resolution of 1.25 x 2 x 2 mm3. Within a 28 second breathhold 64 contiguous 2 mm sectionsare acquired (Table 1). To enable differentiation of free floating fecal material and air bubbles from fixed polypoid lesions, the prone 3D acquisition is repeated following the intravenous administration of 0.1 mmol/kg BW Gd-DTPA (Magnevist, Schering AG, Berlin, FRG). Subsequently the patient is imaged in the supine position. Analysis is based on coronal source-, maximum intensity projection-(MIP), and multiplanar reconstruction (MPR) images, as well as virtual colonoscopy (VC) videos.
Preliminary results:
‘In vitro’ the sensitivity/specificity for the detection of polyps based upon endoscopic MR was 87/96% (4). This performance significantly exceeded the results obtained when analysis was based on exosciopic inspection of the individual cross-sectional images alone.
Careful analysis of 132 ‘in vivo’ patient data sets revealed various pathologies, including masses, diverticula and inflammatory changes (5,6). Virtual endoscopic images allowed visualisation of the colonic haustra as well as most intraluminal mass lesions. Analysis of the 132 patients revealed the following preliminary in vivo results: Polyps under 5 mm in size were only very incompletly (21%) visualised with MRC. The sensitivity for detecting polyps exceeding 10 mm was 93% with a specificity of 99% and a negative predictive value of 98% (Table 2).
Assessment of the colon, particularly with endoscopic viewing, was limited by the presence of air. In all evaluated patients, the initial prone data set provided endoluminal views of most of the colon. Due to the air’s movement to the highest location, small segments, that were not assessable on the prone images due to the presence of air were seen well in the data sets acquired in the supine position. The administration of intravenous contrast further aided in the differentiation of air from polyps. Enhancement was visible in all colonic mass lesions. Contrast-enhanced imaging also allowed for the assessment of the liver in patients with lesions suspicious for malignancy.
Interpretation
3D MRI constitutes an interesting new means for assessing various colonic pathologies. The potential of this technique to provide accurate, minimally invasive, cost efficient polyp screening as well as comprehensive colonic tumor staging warrants further investigation.
References
Tables
Table 1: |
||||||
MR-Colonography (MRC): imaging protocol |
||||||
| patient position | sequences | TR/TE/flip ms/ms/° | slicethk. [mm] | matrix | FOV [cm] | acqui. time [sec.] |
| prone | SPGR | 4.9/1.3/60° | -* | 256x224 | 48 | 1 / image |
| prone | 3D-SPGR | 4.0/2.5/25° | 2.0-2.6 | 384x192 | 36-44 | 30 |
| prone | 2D-SSFSE | -/65/90° | 6 | 256x192 | 36-44 | 28 |
| supine | 3D-SPGR | 4.0/2.5/25° | 2.0-3.0 | 256x160 | 36-44 | 26 |
* non section-selective
Table 2a: |
||||
Diagnostic performance of MR-colonography in 117 patients |
||||
overall and relative to polyp cut-off sizes |
||||
| max. lesion size | all lesions | > 5 mm | >7 mm | >10 mm |
| Sensitivity | 46% | 62% | 75% | 93% |
| Specificity | 81% | 91% | 92% | 99% |
| PPV* | 71% | 69% | 72% | 92% |
| NPV** | 61% | 88% | 93% | 98% |
*PPV = positive predictive value
**NPV = negative predictive value
Table 2b: |
||||
Diagnostic performance of MR-colonography in 702 segments |
||||
overall and relative to polyp cut-off sizes |
||||
| max. lesion size | all lesions | > 5 mm | >7 mm | >10 mm |
| sensitivity | 35% | 64% | 74% | 94% |
| specificity | 96% | 98% | 98% | 99% |
| PPV* | 62% | 76% | 78% | 94% |
| NPV** | 90% | 97% | 98% | 99% |
*PPV = positive predictive value
**NPV = negative predictive value
Figures

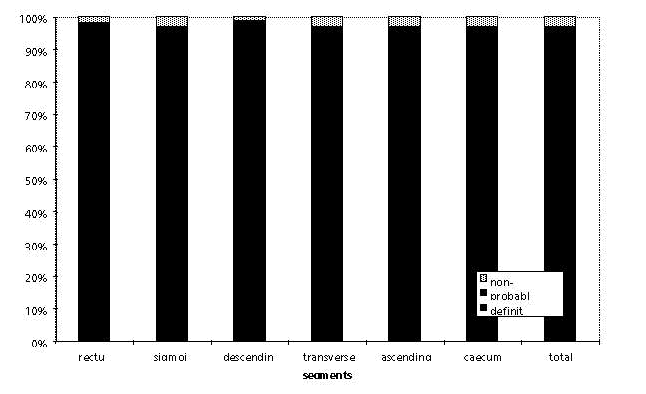
Fig. 1: Summary of ratings (definite, probable, non-diagnostic) provides a reflection of image quality for the various colonic segments.
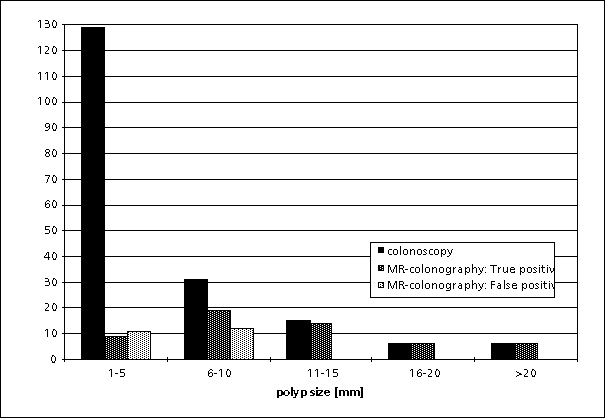
Fig. 2: Detection of colorectal mass lesions with MR-colonography relative to colonoscopy for different size ranges. The performance of MR-colonography improves with increasing mass size.
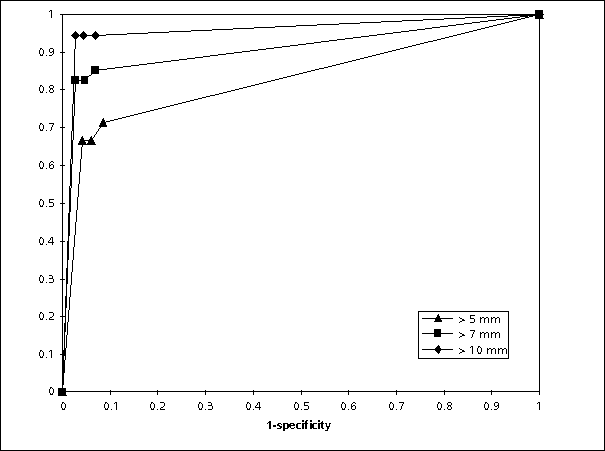
Fig. 4 : ROC-curve illustrating the influence of polyp size on the diagnostic confidendence of MR-colonography. Non-diagnostic exams were included in this analysis and the cut off (> 5mm, > 7 mm, > 10 mm) was only applied to the standard of reference.- Larger polyps were diagnosed with a high confidence almost independent of the underlying image quality.